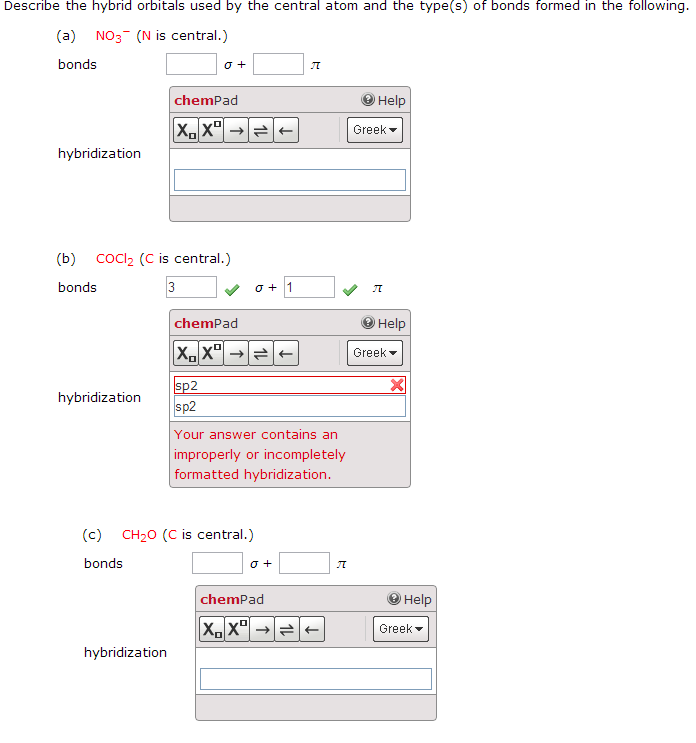
Describe the Hybrid Orbitals Used by the Central Atom I3-
14 Why are d-orbitals sometimes used to form hybrid orbitals. Thus the number of lone pairs on the central iodide atom 3 Also the number of sigma σ bonds 2 and the number of Pi π bonds 0 Now the hybridization of this molecule can be determined by the steric number.
Solved Describe The Hybrid Orbitals Used By The Central Atom Chegg Com
Get solutions Get solutions done loading.

. All the three hybrid orbitals remain in one plane and make an angle of 120 with one another. The molecules in which the central atom is linked to 3 atoms and is sp2 hybridized have a triangular planar shape. While there are 3 Iodine atoms one of the atoms has a negative charge which further gives 2 bond pairs and 3 lone pairs of electrons.
Describe the hybrid orbitals used by the central atom and the numbers of bonds sigma and pi formed in O3. Solution for Describe the hybrid orbitals used by the central atom and the types of bonds formed in a NO₃. Describe the hybrid orbitals used by the central atom and the types of bonds formed ina O3b I3c COCl2 C is central.
Its steric number will be 5. Determine the electron pair geometry using the VSEPR model. The central atom exhibits sp2 hybridization since there is trigonal planar electron pair geometry.
Up to 256 cash back What hybrid orbitals are used by the central atom in the followingexpanded octet species. Knowing the steric number will also help in determining the count of hybrid orbitals used by the atom. Sulphur will use five orbitals including one 3s-orbital three 3p-orbitals and one 3d-orbital.
C COCI2 C is central. 10 How do you identify orbitals. The notion of hybrid orbitals was invented by Linus Pauling in the 1930s as a way of explaining the geometry of molecules primarily the geometry of carbon compounds.
This problem has been solved. 15 What is D bonding. 9 What are the orbitals used for the formation of the CH bonds in the ethene.
The Molecular Geometry of I3- is linear. Principles of General Chemistry 2nd Edition Edit edition. Describe the hybrid orbitals used by the central atoms and the types of bonds formed in SO2.
For each draw an orbital diagram clearly showing theorbitals used and the number of electrons in each orbital. Describe the hybrid orbitals used by the central atom and the type s of bonds formed in a O3. 11 What atomic orbitals are used to make the bond between CA and CB.
Describe the hybrid orbitals used by the central atom and the types of bonds formed in I3. Draw the Lewis structure. Start by drawing the Lewis diagram for SO2.
Ill assume you already know how to do this. C COCl2 C is central. 13 What shape are DXY orbitals.
16 Can sp orbitals. The central sulphur atom. Steps in predicting the hybrid orbitals used by an atom in bonding.
Examples of sp 2 Hybridization. Two double bonds and one lone pair of electrons. Describe the hybrid orbitals used by the central atom and the types of bonds formed in o3.
Solutions for Chapter 11 Problem 22P. The 3 lone pairs will repel each other and take up the equatorial positions. 0706 Which types of atomic orbitals of the central atom mix to form hybrid orbita.
Chemistry questions and answers. Now the hybridization of this molecule can be determined by the steric number. Describe the hybrid orbitals used by the central atom and the types of bonds formed ina O3b I3c COCl2 C is central.
Steric number number of σ bonds number of lone pairs 2 3 5 sp³d hybridization. Also the number of sigma σ bonds 2. When we know the molecular geometry we can use the concept of hybridization to describe the electronic orbitals used by the central atom in bonding.
The Molecular Nature of Matter and Change 5th Edition Edit edition Solutions for Chapter 11 Problem 22P. Principles of General Chemistry 1st Edition Edit edition Solutions for Chapter 11 Problem 22P. Each of the hybrid orbitals formed has a 3333 s character and 6666 p character.
Be sure to answer all parts. And the number of Pi π bonds 0. In 2P-orbitals four hybrid orbitals are overlapped and the fifth one contains a lone pair.
Describe the hybrid orbitals used by the central atom s and the type s of bonds formed in SO2. Be sure to answer all parts. Describe the hybrid orbitals used by the central atom and the types of bonds formed in a O3 b I3-.
Thus the number of lone pairs on the central iodide atom 3. Describe the hybrid orbitals used by the central atom and the type s of bonds formed in I3. There are three electron groups surrounding the central sulfur atom.
Find step-by-step Chemistry solutions and your answer to the following textbook question. 1023 Which types of atomic orbitals of the central atom mix to form hybrid orbita. 12 How do orbitals hybridize.
Important Points To Remember. Steric number number of σ bonds number of lone pairs 2 3 5 sp³d hybridization. If the electron pair geometry is linear the hybridization is sp.
Generally the number of. Describe the hybrid orbitals used by the central atoms and the types of. Describe the hybrid orbitals used by the central atoms and the types of.

Solved Be Sure To Answer All Parts Describe The Hybrid Chegg Com
Comments
Post a Comment